Controversial terms: Difference between revisions
No edit summary |
|||
| Line 6: | Line 6: | ||
:[[Collisionally Activated Dissociation]] | :[[Collisionally Activated Dissociation]] | ||
==In the literature== | |||
A search for occurrences (in 2005) of the two terms in the literature reveals a distinct preference. | |||
Collision-induced dissociation (CID) and collisionally activated dissociation (CAD) refer to the process in which a collision between and ion and a neutral species results in the conversion of part of the translational energy into internal energy of the ion and subsequent fragmentation. The IUPAC document defines the two terms equivalently as does Price (JASMS, 2, 336, 1991). The ASMS Terms and Definitions document does not mention CAD. Sparkman defines CAD and CID equivalently, but notes his preference for CAD. | |||
[[Image:CID_CAD.gif|center]] | |||
A search of the literature for "collision induced dissociation" and "collisionally activated dissociation" suggests that the former term is preferred. In Figure 1, the number of occurrences of the above strings in journal articles is plotted as a function of the year of publication. The plot shows a clear preference for CID over CAD that increases after 1990. This trend can be seen clearly in Figure 2. The occurrence ratio is about 5 in the 80s and early 90s, then jumps to about 30 in the late 90s. | |||
[[Image:CID_CAD_ratio.gif|center]] | |||
Based on this data, should the IUPAC document list collision induced dissociation/CID as the preferred term? | |||
===Google fight=== | ===Google fight=== | ||
Revision as of 15:55, 10 July 2009
Collision Induced Dissociation vs. Collisionally Activated Dissociation
Should CAD be replaced in all cases by CID?
In the literature
A search for occurrences (in 2005) of the two terms in the literature reveals a distinct preference.
Collision-induced dissociation (CID) and collisionally activated dissociation (CAD) refer to the process in which a collision between and ion and a neutral species results in the conversion of part of the translational energy into internal energy of the ion and subsequent fragmentation. The IUPAC document defines the two terms equivalently as does Price (JASMS, 2, 336, 1991). The ASMS Terms and Definitions document does not mention CAD. Sparkman defines CAD and CID equivalently, but notes his preference for CAD.
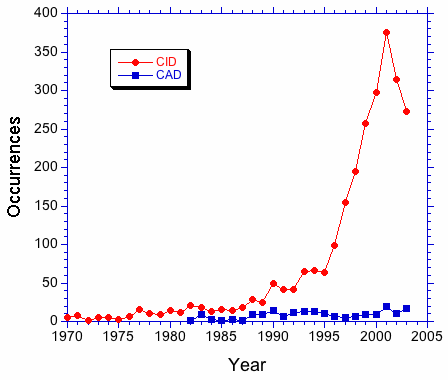
A search of the literature for "collision induced dissociation" and "collisionally activated dissociation" suggests that the former term is preferred. In Figure 1, the number of occurrences of the above strings in journal articles is plotted as a function of the year of publication. The plot shows a clear preference for CID over CAD that increases after 1990. This trend can be seen clearly in Figure 2. The occurrence ratio is about 5 in the 80s and early 90s, then jumps to about 30 in the late 90s.
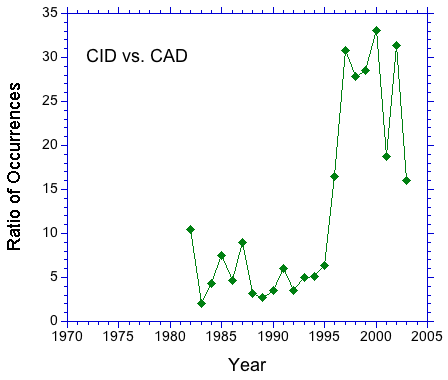
Based on this data, should the IUPAC document list collision induced dissociation/CID as the preferred term?
Google fight
Which is the more widely used term per Google: collision induced dissociation vs collisionally activated dissociation (CID usually wins).
Mass Resolution vs. Mass Resolving Power
How should Resolution and Resolving Power be defined?
Mass-to-Charge Ratio
Should the Thomson be used instead of m/z?
Should be m/z be replaced by m/q?
Parent/Daughter vs. Precursor/Product
Should Parent Ion/Daughter Ion be replaced with Precursor Ion/Product Ion? How about nth generation products?
Slashes and Hyphens
How should Slashes and Hyphens be used in combined techniques?
