Aston 1922/Chapter 2: Difference between revisions
No edit summary |
|||
| (17 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
<big><big>'''Chapter II - The Radioactive Isotopes'''</big></big> | |||
{{Template:Aston 1922 Contents}} | |||
==5. Chemical identities among the radioactive elements== | ==5. Chemical identities among the radioactive elements== | ||
Apart from the purely speculative considerations | Apart from the purely speculative considerations which have aheady been detailed, the theory of isotopes had its birth in the gigantic forward wave of human knowledge inaugurated by the discovery of radioactivity. It can admittedly be argued that, even if no radioactive elements existed, isotopes would inevitably have been discovered by the method of positive rays. But progress must then have been exceedingly slow, and the arrival at the real interpretation of the idea, depending as it does on Sir [[wikipedia:Ernest Rutherford|Ernest Rutherford]]'s theory of the " nucleus" atom, almost impossible. | ||
which have aheady been detailed, the theory of isotopes had | |||
its birth in the gigantic forward wave of human knowledge | |||
inaugurated by the discovery of radioactivity. It can | |||
isotopes would inevitably have been discovered by the method | |||
of positive rays. But progress must then have been | |||
depending as it does on Sir [[wikipedia:Ernest Rutherford|Ernest Rutherford]]'s theory of | |||
the " nucleus" atom, almost impossible. | |||
In 1906 [[wikipedia:Bertram Boltwood|Boltwood]] at Yale discovered a new element in the | In 1906 [[wikipedia:Bertram Boltwood|Boltwood]] at Yale discovered a new element in the radioactive group which he called [[wiktionary:ionium|Ionium]],<ref>Boltwood, ''Amer. J. Sci'', '''22''', 537, 1906;[https://www.proquest.com/docview/89598254?pq-origsite=gscholar&fromopenview=true&sourcetype=Scholarly%20Journals] '''24''', 370, 1907.[https://www.proquest.com/docview/89598004/fulltextPDF/1D7013FDBDF94219PQ/1?accountid=12154&sourcetype=Scholarly%20Journals]</ref> and described as having chemical properties similar to those of thorium. So much was this the case that if, by accident, salts of these two elements were mixed, he found it impossible to separate them again by any of the chemical processes. | ||
radioactive group which he called [[wiktionary:ionium|Ionium]],<ref>Boltwood, ''Amer. J. Sci'', '''22''', 537, 1906;[https://www.proquest.com/docview/89598254?pq-origsite=gscholar&fromopenview=true&sourcetype=Scholarly%20Journals] '''24''', 370, 1907.[https://www.proquest.com/docview/89598004/fulltextPDF/1D7013FDBDF94219PQ/1?accountid=12154&sourcetype=Scholarly%20Journals]</ref> and described | |||
as having chemical properties similar to those of thorium. | |||
So much was this the case that if, by accident, salts of these | |||
two elements were mixed, he found it impossible to separate | |||
them again by any of the chemical processes. | |||
Boltwood, being occupied in the experimental proof that | Boltwood, being occupied in the experimental proof that ionium was the parent substance of radium, did not pursue this line of investigation further at the time, but the work was later taken in hand by Marckwald and Keetman of Berlin.<ref>Keetman, ''Jahr. Radioactivitat'', '''6''', 269, 1909.</ref> Thanks to the rapid advance in radioactive methods there were now at command means of detecting change in concentration of a delicacy unheard of in the previous work on the rare earths, but yet, after years of patient and laborious work, not the slightest sign of separation of ionium and thorium could be observed. The chemical similarity between these two bodies was therefore of an order entirely different to that exhibited by the rare earth elements, and came as near absolute identity as the most critical mind could require. | ||
ionium was the parent substance of radium, did not pursue | |||
this line of investigation further at the time, but the work | |||
was later taken in hand by Marckwald and Keetman of Berlin.<ref>Keetman, ''Jahr. Radioactivitat'', '''6''', 269, 1909.</ref> Thanks to the rapid advance in radioactive methods there | |||
were now at command means of detecting change in concentration of a delicacy unheard of in the previous work on the | |||
rare earths, but yet, after years of patient and laborious work, | |||
not the slightest sign of separation of ionium and thorium | |||
could be | |||
two bodies was therefore of an order entirely different to that | |||
exhibited by the rare earth elements, and came as near absolute | |||
identity as the most critical mind could require. | |||
This result was confirmed in the most rigorous manner by | This result was confirmed in the most rigorous manner by [[wikipedia:Carl Auer von Welsbach|Auer v. Welsbach]]<ref>A. von Welsbach, ''Wien. Ber.'' iia, 119, 1011, 1910.</ref> who was able to apply to the problem his valuable experience in work on the rare earths. | ||
[[wikipedia:Carl Auer von Welsbach|Auer v. Welsbach]]<ref>A. von Welsbach, ''Wien. Ber.'' iia, 119, 1011, | |||
his valuable experience in work on the rare earths. | |||
Furthermore, [[wiktionary:mesothorium|Mesothorium]], discovered by Hahn in 1907, | Furthermore, [[wiktionary:mesothorium|Mesothorium]], discovered by Hahn in 1907, was shown to be chemically inseparable from radium by Marckwald<ref>Marckwald, ''Ber. d. Chem. Oes.'', '''40''', 3420, 1910.</ref> and Soddy<ref>Soddy, ''Trans. Chem. Soc'', '''99''', 72, 1911.</ref> and similar chemical identities were shown to be exceedingly probable in many other cases of radioactive products. Certain regularities in the occurrence of these were pointed out by [[wikipedia:Otto Hahn|Hahn]] and [[wikipedia:Lise Meitner|Meitner]].<ref>Hahn and Meitner, ''Physikal. Zeitsch.'', '''11''', 493, 1910.</ref> | ||
was shown to be chemically inseparable from radium by | |||
Marckwald<ref>Marckwald, ''Ber. d. Chem. Oes.'', '''40''', 3420, 1910.</ref> and Soddy<ref>Soddy, ''Trans. Chem. Soc'', '''99''', 72, 1911.</ref> and similar chemical identities were | |||
shown to be exceedingly probable in many other cases of | |||
radioactive products. Certain regularities in the occurrence | |||
of these were pointed out by [[wikipedia:Otto Hahn|Hahn]] and [[wikipedia:Lise Meitner|Meitner]].<ref>Hahn and Meitner, ''Physikal. Zeitsch.'', '''11''', 493, 1910.</ref> | |||
The situation was admirably summed up by Soddy in his | The situation was admirably summed up by Soddy in his report on radioactivity for the year 1910<ref>Soddy, ''Chem. Soc. Ann. Rep.'', '''285''', 1910.</ref> in the following words: | ||
report on radioactivity for the year 1910<ref>Soddy, ''Chem. Soc. Ann. Rep.'', '''285''', 1910.</ref> in the following words: | |||
"These regularities may prove to be the beginning of some | <blockquote>"These regularities may prove to be the beginning of some embracing generalisation, which will throw light, not only on radioactive processes, but on elements in general and the Periodic Law. Of course, the evidence of chemical identity is not of equal weight for all the preceding cases, but the complete identity of ionium, thorium and radiothorium, of radium and mesothorium 1, of lead and radium D, may be considered thoroughly established. . . . The recognition that elements of different atomic weights may possess identical properties seems destined to have its most important apphcation in the region of inactive elements, where the absence of a second radioactive nature makes it impossible for chemical identity to be individually detected. Chemical homogeneity is no longer a guarantee that any supposed element is not a mixture of several of different atomic weights, or that any atomic weight is not merely a mean number. The constancy of atomic weight, whatever the source of the material, is not a complete proof of homogeneity, for, as in the radioelements, genetic relationships might have resulted in an initial constancy of proportion between the several individuals, which no subsequent natural or artificial chemical process would be able to disturb. If this is the case, the absence of simple numerical relationships between the atomic weights becomes a matter of course rather than one of surprise."</blockquote> | ||
embracing generalisation, which will throw light, not only on | |||
radioactive processes, but on elements in general and the | |||
Periodic Law. Of course, the evidence of chemical identity | |||
is not of equal weight for all the preceding cases, but the | |||
complete identity of ionium, thorium and radiothorium, of | |||
radium and mesothorium 1, of lead and radium D, may be | |||
considered thoroughly established. . . . The recognition | |||
that elements of different atomic weights may possess identical | |||
properties seems destined to have its most important | |||
a second radioactive nature makes it impossible for chemical | |||
identity to be individually detected. Chemical homogeneity | |||
is no longer a guarantee that any supposed element is not a | |||
mixture of several of different atomic weights, or that any | |||
atomic weight is not merely a mean number. The constancy | |||
of atomic weight, whatever the source of the material, is not | |||
a complete proof of homogeneity, for, as in the radioelements, | |||
genetic relationships might have resulted in an initial constancy | |||
of proportion between the several individuals, which no subsequent natural or artificial chemical process would be able to | |||
disturb. If this is the case, the absence of simple numerical | |||
relationships between the atomic weights becomes a matter of | |||
course rather than one of surprise." | |||
==6. Spectroscopic identity of isotopes== | |||
The next great advance was of an even more revolutionary character. This consisted in the demonstration that the chemically indistinguishable products of the transformation of the radioactive elements might also be spectroscopically identical. The idea that elements of different atomic weight might yet have the same spectrum originated in Sir Ernest Rutherford's laboratory and appears to have been first entertained by [https://www.nature.com/articles/237120a0.pdf A. S. Russell]. With Rossi<ref>Russell and Rossi, ''Proc. Roy. Soc'', '''77A''', 478, 1912.</ref> he undertook the comparison between the spectrum of pure thorium and that of a mixture of thorium and ionium which radioactive evidence showed to contain a large percentage of the latter element. No new fines attributable to ionium were observed; in fact the spectra obtained were absolutely indistinguishable. | |||
percentage | After giving in full the radioactive evidence as to the probable percentage of ionium present, and showing that it was practically impossible for this to be too small for its spectrum to appear, the writers go on as follows: | ||
<blockquote>"There are, however, two other possible ways of explaining our failure to obtain a distinct spectrum for ionium, besides the one discussed above. It is possible that : | |||
" | "(1) Ionium has no arc spectrum in the region investigated, | ||
or | or | ||
" | "(2) Ionium and thorium have identical spectra in the region investigated. | ||
investigated. | |||
"The first possibility is highly improbable, for all solids of high atomic weights have arc spectra, and, further, aU rare earths have highly complicated spectra. | |||
"The second possibility, though somewhat speculative in nature, is suggested by some recent work on the chemical properties of the radio-elements. There is no evidence at present to disprove its truth. It is well known that there are no less than four sets of longlived radio-elements, the members of each of which are chemically non-separable. These elements do not all belong to the group of rare earths, many non-radioactive members of which are known to be chemically very similar. Mesothorium, for instance, which is chemically non-separable ' from radium, belongs to the alkaline earth group. Again the two non-separable a ray products which are present in ordinary uranium, and which have been called by Geiger and Nuttall uranium I and uranium II belong to the chromium-molybdenum-tungsten group of elements. The explanation of these striking chemical similarities is very probably that the two very similar bodies are really different members of the same group of elements, the difference in their chemical properties being less pronounced than the difference between other members of the same group, owing to the small difference in their atomic weights. But the possibility that they are identical in all physical and chemical properties, and differ only in atomic weight and in radioactive properties, should not be lost sight of. If this explanation should eventually prove justified, the spectrum of ionium would he identical with that of thorium."</blockquote> | |||
It is not surprising that the idea was put forward with some caution. Unlike that of chemical identity which had been led up to by a gradual series of steps, it was entirely new and contrary to all the preconceived ideas of the relations between the spectrum of an element and the masses of its atoms. The new departure was supported by Soddy<ref>Soddy, ''Chem. News'', Feb. 28, 1913.</ref> but received some adverse criticism on the ground of insufficient evidence. The later work bearing on this point will be described in Chapter X. | |||
Already in 1911 the theory of the "Nucleus Atom"<ref>V. p. 92.</ref> had been formulated. This gave the first hint as to the physical meaning of chemical and spectroscopic identity, namely that the nuclei of atoms might vary in their mass but yet, at the same time, possess some property in common with each other, namely nuclear charge, upon which the chemistry and spectra depend. | |||
In 1912 appeared the electrochemical work of [[wikipedia:George de Hevesy|Hevesy]],<ref>G. Hevesy, ''Phil. Mag.'', 23, 628, 1912 ; ''Physikal. Zeitsch.'' '''15''', 672, 715, 1912.</ref> which led to the discovery of the remarkable field of research opened up by the use of radioactive bodies as indicators.<ref>V. p. 19.</ref> A little later [[wikipedia:Friedrich Paneth|Paneth]] and Hevesy were able to show the complete identity of the electrochemical properties of [[wikipedia:Isotopes of lead|Radium D]] and Lead.<ref>Paneth and Hevesy, Sitzungber. K. Akad. Wiss. Wien, iiA, 123, 1037, 1913. </ref> | |||
In 1914 Rutherford and [[wikipedia:Edward Andrade|Andrade]]<ref>Rutherford and Andrade, Phil. Mag. 27, 854, 1914. </ref> examined the self-excited X-ray spectrum of [[wikipedia:Polonium-210|radium B]]. They used a crystal of rock salt for the analysis and got rid of the effect of the swift β rays by putting the source in a strong magnetic field. The wave length of the L radiation proved to be exactly that expected for lead from Moseley's experiment.<ref>V. p. 93. </ref> This was the first proof that isotopes had identical X-ray spectra. The actual values for ordinary lead were subsequently determined by [[wikipedia:Manne Siegbahn|Siegbahn and found to be in excellent agreement with Rutherford and Andrade's results. | |||
==7. The Chemical Law of Radioactive change== | |||
This law, put in the briefest form, asserts: A radioactive element when it loses an alpha particle goes back two places in the Periodic Table; when it loses a beta particle it goes forward one place. | |||
The law has been associated with the name of Soddy<ref>V. Stewart, ''Recent Advances in Physical and Inorganic Chemistry'', Longmans, 1919.</ref> who was the first to suggest, in the form of a valency property, that part of it relating to alpha rays.<ref>Soddy, ''The Chemistry of the Radio Elements'', 29, First Edition, Longmans, 1911. </ref> But in its more complete enunciation, which took place early in 1913, at least four other investigators can claim a share. | |||
Russell was the first to publish a law covering both Kinds of rays,<ref>Russell, ''Chem. News,'' Jan, 31, 1913, </ref> but owing to the fact that he failed to realise that the sequence of elements in the periodic table is a continuous expression, his statement was not so simple and definite as it might have been. [[wikipedia:Kazimierz Fajans|Fajans]],<ref>Fajans, ''Physikal. Zeitsch.'' Feb. 15, 1913. </ref> using as foundation the electrochemical results of [[wikipedia:George de Hevesy|Hevesy]],<ref>Hevesy, ''Physikal. Zeitsch.'' Jan. 15, 1913. </ref> and Soddy, working on the results of a very full chemical investigation carried out at his request by Fleck,<ref>Fleck, Trans. Chem. Soc. 103, 381, 1052, 1913. </ref> published the generalisation in its full and complete form independently, and practically at the same time. | |||
This law, which will be shown later to be a natural consequence of the much wider generalisation discovered by Moseley,<ref>V. p. 93. </ref> has been of the greatest value in correlating the numerous products of radioactive change, and predicting with accuracy which of them mil have identical properties. To the latter the name Isotopes was appled by Soddy in the following words: "The same algebraic sum of the positive and negative charges in the nucleus when the arithmetical sum is different gives what I call 'isotopes' or 'isotopic elements' because they occupy the same place in the periodic table. They are chemically identical, and save only as regards the relatively few physical properties which depend upon atomic mass directly, physically identical also." Any element which is the result of a series of changes involving the loss of twice as many beta particles as alpha particles must clearly be the isotope of the parent element, since it must inevitably, by the above law, reach the same place in the periodic table at the end of these operations. | |||
==8. Isobares== | |||
Just as we can have elements of the same chemical properties but different atomic weight so we can also have those with the same atomic weight but different chemical properties. These [[wikipedia:Alfred Walter Stewart|Stewart]] <ref>Stewart, ''Phil. Mag.'' '''36''', 326, 1918.[{{doi}}10.1080/14786441008635836] </ref> has called "[[Isobar]]es." Any product due to the loss of a [[wikipedia:Beta particle|beta ray]] (which has a negligible mass)<ref>V. p. 91. </ref> must be an isobare of its parent substance, for, without change of mass, it has moved in the periodic table and so changed its chemical properties. It is interesting to note in this connection that no isobare has actually been discovered among the non-radioactive elements as yet, but they must certainly exist.<ref>V. p. 77. </ref> | |||
==9. The Radioactive Transformations== | |||
[[File:Aston 1922 Figure 1.jpg|thumb|400 px|Fig. 1. Diagram of the transformations of the radio-elements showing atomic number, atomic weight and period of disintegration of each product.]] The radioactive elements are all formed from the two parent elements inanium and thorium by a series of changes or transformations. These changes can be classified according to their nature into two types. In the first type of change called the α ray change the atom loses a particle of mass 4 carrying two positive charges {+2''e'') which has been identified with the nucleus of the helium atom.^<ref>V. Table p. 106. </ref> In the second or β ray change the particle shot off has a negligible mass and carries a single negative charge (–e). Hence in an a ray change the element loses 4 units in atomic weight, while in a β ray change its weight is unaltered. | |||
The rate of decay of an element is measured by the " half value " period which may vary from 10<sup>10</sup> years to 10<sup>-11</sup> of a second. The velocity with which the rays are ejected also varies and is apparently connected with the period of the element by the very interesting relation of [[wikipedia:Geiger–Nuttall law|Geiger and Nuttall.<ref>Rutherford, Radioactive Substances and their Radiations, p. 607, Cambridge, 1913. </ref> The intricate researches by which the complex series of transformations have been explained belong to the subject of Radioactivity and cannot be described here. From the point of view of isotopes it will be enough to consider the final results which are given in the two diagrams (Figs. 1 and 2). | |||
In the first of these, which is due to Soddy,<ref>Soddy, Trans. Chem. Soc, U5, 16, 1919.</ref> the nuclear charge or Atomic number,<ref>V. p. 93. </ref> upon which all the chemical and spectroscopic properties of the elements depend, and which expresses its position in the periodic table, is indicated by a series of columns edged with thick fines sloping downwards to the right. The atomic weights are shown by fine lines sloping in the opposite direction. The fines corresponding to even atomic weights have been omitted to simplify the scheme. All elements lying in the same column will therefore be ''isotopes'' and all elements lying on the same fine sloping up to the right will be ''isobares''. The α and β ray changes are shown by arrows and the period of decay of the elements indicated by times expressed in suitable units. | |||
[[File:Aston 1922 Figure 2.jpg|thumb| | [[File:Aston 1922 Figure 2.jpg|thumb|400 px|left|Fig. 2. Diagram of the radioactive transformations in relation to atomic numbers. In every case a step two downwards is accompanied by the emission of an a particle and one downwards by a β particle.]] | ||
numbers. | |||
emission | |||
and | The second diagram<ref>Darwin, ''Nature'', '''106''', 82, 1920.[https://archive.org/details/naturejournal106londuoft/page/82/mode/1up]</ref> is arranged in a simple manner to show the general chains of transformation at a glance. In it the α and βray changes are plotted against atomic number and the other information omitted. On this diagram all elements lying on the same horizontal level will be isotopes. To take an example, uranium I which has an atomic number 92 and an atomic weight 238 loses one α particle and becomes [[wikipedia:Protactinium|uranium X]], atomic number 90, atomic weight 234, This then gives off two β rays in succession, first becoming [[wikipedia:Isotopes of protactinium|uranium X2]] and then [[wikipedia:Isotopes of uranium|Uranium II]]. Uranium II has an atomic number 92 so that it is an isotope of uranium I. It has an atomic weight 234 so it is anisobare of uranium Xi and uranium X2. Uranium II can disintegrate by shooting off an a particle in two different ways ; about 8 per cent, of its atoms appear to form uranium Y, which is probably the parent substance of the actinium series. Disregarding this for the moment and following the main chain, 92 per cent, of the atoms of uranium II suffer an α ray change and are transformed into ionium, atomic weight 230, atomic number 90. Ionium loses an a particle and becomes radium, atomic weight 226. This by the same process changes to radium emanation, then to radium A, and then to radium B with atomic weight 214, We see that uranium II has lost 5 α particles in succession, thereby coming back 10 places, 92-82 in the periodic table, and its atomic weight has been reduced 20 units in the process. Radium B loses a β particle, becoming radium C which can disintegrate in two different ways. An extremely small proportion, 0.03 per cent., of its atoms undergo an α ray change to radium C2 which then loses a β particle and may become inactive lead of atomic weight 210. The vast majority of the atoms of radium C lose a β particle and form radium C. This next loses an a particle and becomes radium D, an active isotope of lead of atomic weight 210. Radium D now loses two β particles in succession, becoming radium E and then radium E, which is also called [[wikipedia:polonium|polonium]]. This finally undergoes its last a ray change and becomes inactive uranium lead of atomic weight 206. | ||
92 | |||
weight | |||
Uranium | |||
two | |||
The thorium and actinium chains can be followed on the diagrams in the same manner, but in the case of actinium the parent elements are not satisfactorily settled so that the atomic weights in this series are all doubtful. | |||
the | |||
==10. The Atomic Weight of Lead== | |||
The theory of Isotopes of which Professor Soddy had proved himself so prominent an advocate and defender, received its most triumphant vindication, as far as it concerned the products of radioactivity, at the hands of the very chemists who had most reason to doubt its general apphcation, the specialists in the determination of atomic weights. | |||
The charts of radioactive disintegration<ref>P. 14.</ref> show that the final product of every series is lead. If we take the main chain of the uranium-radium transformation this lead must have an atomic weight 206, for it has lost 5 alpha particles – each of weight 4 – since it was radium, and the atomic weight of radium is 226. On the other hand if we take the main thorium chain the lead end product must be 6 alpha particles lighter than thorium (232.15) and so should have an atomic weight about 208. | |||
Now ordinary lead, from non-radioactive sources has an atomic weight 207.20, so Soddy<ref>1 Soddy, ''Ann. Rep. Chem. Soc'', '''269''', 1913.</ref> suggested in 1913 that the lead derived from minerals containing uranium but no thorium might have a smaller atomic weight than ordinary lead, and on the other hand the atomic weight of lead from minerals containing thorium but no uranium might be greater. | |||
The first experiments were made by Soddy and Hyman<ref>2 Soddy and Hyman, ''Trans. Chem. Soc'', '''105''', 1402, 1914.</ref> with a very small quantity of lead from Ceylon Thorite. This gave a perceptibly higher atomic weight than ordinary lead. Later a large quantity of the same mineral was available. The lead from this when carefully purified gave a density 0'26 per cent, higher than that of common lead. On the assumption that the atomic volumes of isotopes are equal this figure corresponds to an atomic weight of 207.74. A chemical atomic weight determination gave 207-694. A sample of the same lead was sent to Vienna where Professor [[wikipedia:Otto Hönigschmid|Honigschmid]], a well known expert in such matters, obtained from it a value 207.77 as a mean of eight determinations. These figures not only showed that thorium lead had a higher atomic weight than ordinary lead but also that their atomic volumes were identical, as expected from theory.<ref>Soddy, ''Roy. Ins.'', May 18, 1917.</ref> | |||
At the same time as this work was in progress, the leading American authority on atomic weights, [[wikipedia:Theodore William Richards|T. W. Richards]] of Harvard, started a series of investigations on lead derived from various radioactive minerals.<ref>Richards and Lembert, ''J. Amer. Chem. Soc'', ''36'', 1329, 1914. [{{doi}}10.1021/ja02184a001] </ref> The samples of lead from uranium minerals all gave results lower than ordinary lead, as was expected, and one particularly pure specimen of uraniolead from Norwegian cleveite gave 206.08,<ref>5 Richards and Wadsworth, J. Amer. Chem. Soc, 38, 2613, 1916.</ref> a very striking agreement with theory. The following table of properties is taken from his Presidential address to the American Association at Baltimore, December, 1918. | |||
At | |||
American | |||
Harvard, | |||
various | |||
uranium | |||
as | |||
agreement | |||
is | |||
Association | |||
| Line 620: | Line 108: | ||
|} | |} | ||
In | In further confirmation [[wikipedia:Maurice Curie|Maurice Curie]] in Paris<ref>M. Curie, ''Compt. Bend.'', '''158''', 1676, 1914.</ref> reported 206.36 for a lead from carnotite, and a still lower figure, 206.046, was obtained by Honigschmid in Vienna for a lead from the very pure crystalised pitchblende from Morogoro. This is the lowest atomic weight found so far. The highest, 207.9, was also determined by Honigschmid for lead from Norwegian thorite.<ref>Honigschmid, ''Zeit. Elektrochem.'', '''24''', 163, 1918 ; '''25''', 91, 1919.</ref> | ||
206 | |||
046, | |||
was | |||
very | |||
the | |||
was | |||
thorite. | |||
==11. Atomic weights of Thorium and Ionium== | |||
Although the above results obtained with lead are far the most conclusive and important it is not the only element which affords direct experimental evidence of the different atomic weights of isotopes. The atomic weight of ionium, calculated by adding the weight of one alpha particle to the atomic weight of its product, radium, is 230, whereas that of thorium, its isotope, is slightly above 232. Joachimsthal pitchblende contains hardly any thorium so that an ionium-thorium preparation separated by Auer von Welsbach from 30 tons of this mineral might be regarded as containing a maximum concentration of ionium. On the other hand the period of thorium is about 10<sup>5</sup> times longer than that of ionium so that it was doubtful if even in this preparation there would be enough ionium to show a difference in atomic weight. Honigschmid and Mlle. [[wikipedia:Stefanie Horovitz|Horovitz]] have made a special examination of this point, first redetermining as accurately as possible the atomic weight of thorium and then that of the thorium-ionium preparation from pitchblende. They found 232.12 for the atomic weight of thorium, and by the same careful method 231.51 for that of the thorium-ionium. | |||
==12. Use of radioactive isotopes as indicators== | |||
Consider an inactive element A which has a radioactive isotope B. If these are mixed together in any proportions no chemical or physical process known is capable of altering the ratio of the proportions of this mixture to any measurable extent. Now the radioactive methods of detecting and measuring B are many millions of millions of times more delicate than the chemical methods of detecting and measuring A, so that by mixing with A a small quantity of B we can trace its presence far beyond the limits of chemical analysis. We have, as it were, marked the atoms of A with an indelible label so that the minutest trace of the element can be measured with ease and certainty. | |||
By this powerful and novel device, which has been developed by G. Hevesy<ref>Hevesy, ''Brit. Assoc'', 1913 ; ''Chem. News'', Oct. 13, 166, 1913.</ref> 10<sup>-9</sup> gr. of lead can be determined quantitatively and solution concentrations can be dealt with down to 10<sup>-14</sup> of normal. By adding radium D to the lead salt and estimating it electroscopically the solubility of lead sulphide and chromate, and the amount of lead chloride carried down in a silver chloride precipitate, may readily be determined. | |||
Recently, by the same principle, it has been shown that a free exchange of the metallic atom among the competing acid radicles occurs for ionised, but not for non-ionised, compounds. The general method was to mix solutions of two different compounds of lead in equimolecular proportions, the one compound only being "activated" by presence of thorium-B (which is isotopic with lead), and to determine the activity of the lead in the less soluble compound crystallising out. When active lead nitrate and inactive lead chloride are dissolved in molecular proportion in boiling pyridine, the lead in the lead chloride crystalHsing out is half as active as the lead in the original lead nitrate, but when such an active lead salt is so mixed with an organic compound of lead, such as lead tetraphenyl or diphenyl nitrate, in suitable solvents, no interchange of lead occurs, and the active lead salt retains its original activity. This constitutes something like a direct proof of the ionic dissociation theory and of the current views as to the difference between the nature of chemical union in electrolytes and non-electrolytes. When the acetates of quadrivalent activated lead and of bivalent inactive lead are mixed in glacial acetic acid, the activity of the first compound, after crystallising out from the mixture, is reduced to one half. This indicates, since the two lead ions differ only by two electrons, a free interchange of electrons between them and a dynamic equilibrium between ions and electrons and between free electrons and the electrodes in electrolysis.<ref>G. Hevesy and L. Zechmeister, ''Ber''., '''53B''', 410, 1920 ; ''Zeitsch. Elektrochem.'' '''26''', 151, 1920.</ref> | |||
Isotopes can also be used to determine the velocity of diffusion of molecules among themselves.<ref>J. Groh and Hevesy, ''Ann. Physik.'', iv., '''63''', 85, 1920.</ref> The rate of diffusion is dependent on the molecular diameter, and not on the mass, so that a radioactive element diffusing among the inactive molecules of its isotope affords a means of investigating this otherwise insoluble problem. The experiment has been tried with molten lead. At the bottom of a narrow vertical tube was placed a layer of lead rendered active by the presence of thorium-B, and above it a layer three times the height of common lead. The whole was kept at 340° for several days. After cooling, the cylinder was cut into four equal lengths, each melted and hammered into foil, and the concentration of thorium-B in each determined by alpha ray measurements. Values for the diffusion coefficient between 1.77 and 2.54 per sq. cm. per day, with a mean of 222 in seventeen experiments, were obtained. On certain theories of physical chemistry this corresponds with a diameter of the lead molecule between 0.78 and I1.16 x 10<sup>-8</sup> cm., according to the formulae used to connect the two quantities. The value found by similar theories when reduced to a temperature of 18° and for a fluid of the viscosity of water, becomes 2.13. Since the value for lead ions diffusing in aqueous solutions is 0.68, this indicates that the molecular diameter in the case of metallic lead is only a third of that in the case of the ion, and shows that the latter is probably hydrated.<ref>Soddy, Ann. Rep. Chem. Soc, 227, 1920.</ref> | |||
of | |||
the | |||
==13. Classification of the radioactive isotopes== | |||
It is clear that the relations between isotopes formed by radioactive | |||
disintegrations need not necessarily have the same simple form as those subsisting between isotopes of the inactive elements. Neuberger,<ref>Neuburger, Nature, 108, 180, 1921.</ref> using the nucleus model of the radioelements proposed by Lise Meitner,<ref>Meitner, ''Die Naturwissenschaften'', '''9''', 423, 1921. </ref> suggests that the radioactive isotopes may be divided into three or even four classes. | |||
:(1) Isotopes of the first class are those which possess only the same nuclear charge and the same arrangement of outer electrons such as radium and mesothorium I. | |||
:(2) Isotopes of the second class have, in addition, the same nuclear mass, that is to say the same atomic weight, and the same total number of nuclear "building stones." Examples of this class are ionium and uranium Y. | |||
of | |||
:(3) Isotopes of the third class still possess the same number of each nuclear building stone, but they have a different arrangement of these in the atomic nucleus, and thus possess different chances of disintegrating, such as Radium D and Actinium B. | |||
:(4) Isotopes of the fourth class would be those possessing the same arrangement of nuclear building stones in the atomic nucleus, and thus the same probability of disintegrating. Such isotopes actually exist, but we have no available means of distinguishing between them. Hence we cannot at present designate them definitely as isotopes. Examples of these are radium C<sub>2</sub> and actinium D. | |||
=== | ==References== | ||
<references/> | <references/> | ||
---- | |||
{{Template:Aston 1922 Contents}} | |||
Latest revision as of 14:02, 31 July 2025
Chapter II - The Radioactive Isotopes
Francis William Aston (1922), Isotopes, ISBN 978-1016732383, Internet Archive.
5. Chemical identities among the radioactive elements
Apart from the purely speculative considerations which have aheady been detailed, the theory of isotopes had its birth in the gigantic forward wave of human knowledge inaugurated by the discovery of radioactivity. It can admittedly be argued that, even if no radioactive elements existed, isotopes would inevitably have been discovered by the method of positive rays. But progress must then have been exceedingly slow, and the arrival at the real interpretation of the idea, depending as it does on Sir Ernest Rutherford's theory of the " nucleus" atom, almost impossible.
In 1906 Boltwood at Yale discovered a new element in the radioactive group which he called Ionium,[1] and described as having chemical properties similar to those of thorium. So much was this the case that if, by accident, salts of these two elements were mixed, he found it impossible to separate them again by any of the chemical processes.
Boltwood, being occupied in the experimental proof that ionium was the parent substance of radium, did not pursue this line of investigation further at the time, but the work was later taken in hand by Marckwald and Keetman of Berlin.[2] Thanks to the rapid advance in radioactive methods there were now at command means of detecting change in concentration of a delicacy unheard of in the previous work on the rare earths, but yet, after years of patient and laborious work, not the slightest sign of separation of ionium and thorium could be observed. The chemical similarity between these two bodies was therefore of an order entirely different to that exhibited by the rare earth elements, and came as near absolute identity as the most critical mind could require.
This result was confirmed in the most rigorous manner by Auer v. Welsbach[3] who was able to apply to the problem his valuable experience in work on the rare earths.
Furthermore, Mesothorium, discovered by Hahn in 1907, was shown to be chemically inseparable from radium by Marckwald[4] and Soddy[5] and similar chemical identities were shown to be exceedingly probable in many other cases of radioactive products. Certain regularities in the occurrence of these were pointed out by Hahn and Meitner.[6]
The situation was admirably summed up by Soddy in his report on radioactivity for the year 1910[7] in the following words:
"These regularities may prove to be the beginning of some embracing generalisation, which will throw light, not only on radioactive processes, but on elements in general and the Periodic Law. Of course, the evidence of chemical identity is not of equal weight for all the preceding cases, but the complete identity of ionium, thorium and radiothorium, of radium and mesothorium 1, of lead and radium D, may be considered thoroughly established. . . . The recognition that elements of different atomic weights may possess identical properties seems destined to have its most important apphcation in the region of inactive elements, where the absence of a second radioactive nature makes it impossible for chemical identity to be individually detected. Chemical homogeneity is no longer a guarantee that any supposed element is not a mixture of several of different atomic weights, or that any atomic weight is not merely a mean number. The constancy of atomic weight, whatever the source of the material, is not a complete proof of homogeneity, for, as in the radioelements, genetic relationships might have resulted in an initial constancy of proportion between the several individuals, which no subsequent natural or artificial chemical process would be able to disturb. If this is the case, the absence of simple numerical relationships between the atomic weights becomes a matter of course rather than one of surprise."
6. Spectroscopic identity of isotopes
The next great advance was of an even more revolutionary character. This consisted in the demonstration that the chemically indistinguishable products of the transformation of the radioactive elements might also be spectroscopically identical. The idea that elements of different atomic weight might yet have the same spectrum originated in Sir Ernest Rutherford's laboratory and appears to have been first entertained by A. S. Russell. With Rossi[8] he undertook the comparison between the spectrum of pure thorium and that of a mixture of thorium and ionium which radioactive evidence showed to contain a large percentage of the latter element. No new fines attributable to ionium were observed; in fact the spectra obtained were absolutely indistinguishable.
After giving in full the radioactive evidence as to the probable percentage of ionium present, and showing that it was practically impossible for this to be too small for its spectrum to appear, the writers go on as follows:
"There are, however, two other possible ways of explaining our failure to obtain a distinct spectrum for ionium, besides the one discussed above. It is possible that :
"(1) Ionium has no arc spectrum in the region investigated,
or
"(2) Ionium and thorium have identical spectra in the region investigated.
"The first possibility is highly improbable, for all solids of high atomic weights have arc spectra, and, further, aU rare earths have highly complicated spectra.
"The second possibility, though somewhat speculative in nature, is suggested by some recent work on the chemical properties of the radio-elements. There is no evidence at present to disprove its truth. It is well known that there are no less than four sets of longlived radio-elements, the members of each of which are chemically non-separable. These elements do not all belong to the group of rare earths, many non-radioactive members of which are known to be chemically very similar. Mesothorium, for instance, which is chemically non-separable ' from radium, belongs to the alkaline earth group. Again the two non-separable a ray products which are present in ordinary uranium, and which have been called by Geiger and Nuttall uranium I and uranium II belong to the chromium-molybdenum-tungsten group of elements. The explanation of these striking chemical similarities is very probably that the two very similar bodies are really different members of the same group of elements, the difference in their chemical properties being less pronounced than the difference between other members of the same group, owing to the small difference in their atomic weights. But the possibility that they are identical in all physical and chemical properties, and differ only in atomic weight and in radioactive properties, should not be lost sight of. If this explanation should eventually prove justified, the spectrum of ionium would he identical with that of thorium."
It is not surprising that the idea was put forward with some caution. Unlike that of chemical identity which had been led up to by a gradual series of steps, it was entirely new and contrary to all the preconceived ideas of the relations between the spectrum of an element and the masses of its atoms. The new departure was supported by Soddy[9] but received some adverse criticism on the ground of insufficient evidence. The later work bearing on this point will be described in Chapter X.
Already in 1911 the theory of the "Nucleus Atom"[10] had been formulated. This gave the first hint as to the physical meaning of chemical and spectroscopic identity, namely that the nuclei of atoms might vary in their mass but yet, at the same time, possess some property in common with each other, namely nuclear charge, upon which the chemistry and spectra depend.
In 1912 appeared the electrochemical work of Hevesy,[11] which led to the discovery of the remarkable field of research opened up by the use of radioactive bodies as indicators.[12] A little later Paneth and Hevesy were able to show the complete identity of the electrochemical properties of Radium D and Lead.[13]
In 1914 Rutherford and Andrade[14] examined the self-excited X-ray spectrum of radium B. They used a crystal of rock salt for the analysis and got rid of the effect of the swift β rays by putting the source in a strong magnetic field. The wave length of the L radiation proved to be exactly that expected for lead from Moseley's experiment.[15] This was the first proof that isotopes had identical X-ray spectra. The actual values for ordinary lead were subsequently determined by [[wikipedia:Manne Siegbahn|Siegbahn and found to be in excellent agreement with Rutherford and Andrade's results.
7. The Chemical Law of Radioactive change
This law, put in the briefest form, asserts: A radioactive element when it loses an alpha particle goes back two places in the Periodic Table; when it loses a beta particle it goes forward one place.
The law has been associated with the name of Soddy[16] who was the first to suggest, in the form of a valency property, that part of it relating to alpha rays.[17] But in its more complete enunciation, which took place early in 1913, at least four other investigators can claim a share.
Russell was the first to publish a law covering both Kinds of rays,[18] but owing to the fact that he failed to realise that the sequence of elements in the periodic table is a continuous expression, his statement was not so simple and definite as it might have been. Fajans,[19] using as foundation the electrochemical results of Hevesy,[20] and Soddy, working on the results of a very full chemical investigation carried out at his request by Fleck,[21] published the generalisation in its full and complete form independently, and practically at the same time.
This law, which will be shown later to be a natural consequence of the much wider generalisation discovered by Moseley,[22] has been of the greatest value in correlating the numerous products of radioactive change, and predicting with accuracy which of them mil have identical properties. To the latter the name Isotopes was appled by Soddy in the following words: "The same algebraic sum of the positive and negative charges in the nucleus when the arithmetical sum is different gives what I call 'isotopes' or 'isotopic elements' because they occupy the same place in the periodic table. They are chemically identical, and save only as regards the relatively few physical properties which depend upon atomic mass directly, physically identical also." Any element which is the result of a series of changes involving the loss of twice as many beta particles as alpha particles must clearly be the isotope of the parent element, since it must inevitably, by the above law, reach the same place in the periodic table at the end of these operations.
8. Isobares
Just as we can have elements of the same chemical properties but different atomic weight so we can also have those with the same atomic weight but different chemical properties. These Stewart [23] has called "Isobares." Any product due to the loss of a beta ray (which has a negligible mass)[24] must be an isobare of its parent substance, for, without change of mass, it has moved in the periodic table and so changed its chemical properties. It is interesting to note in this connection that no isobare has actually been discovered among the non-radioactive elements as yet, but they must certainly exist.[25]
9. The Radioactive Transformations
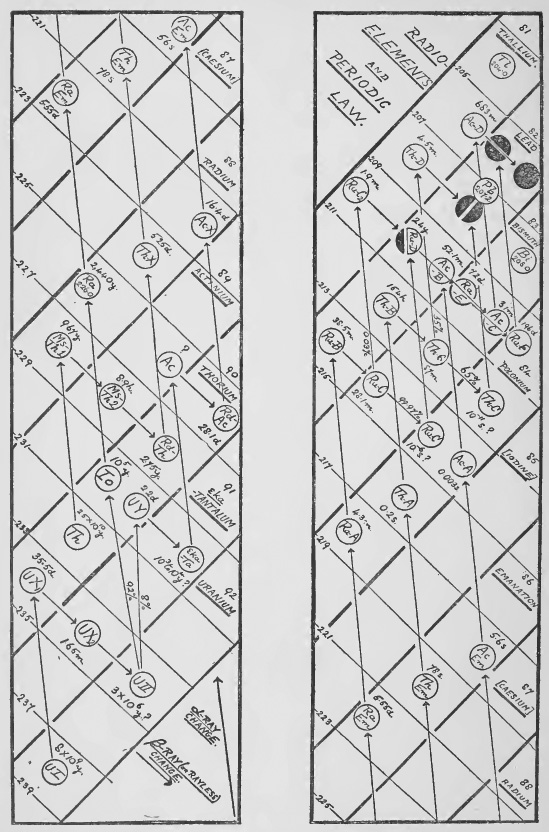
The radioactive elements are all formed from the two parent elements inanium and thorium by a series of changes or transformations. These changes can be classified according to their nature into two types. In the first type of change called the α ray change the atom loses a particle of mass 4 carrying two positive charges {+2e) which has been identified with the nucleus of the helium atom.^[26] In the second or β ray change the particle shot off has a negligible mass and carries a single negative charge (–e). Hence in an a ray change the element loses 4 units in atomic weight, while in a β ray change its weight is unaltered.
The rate of decay of an element is measured by the " half value " period which may vary from 1010 years to 10-11 of a second. The velocity with which the rays are ejected also varies and is apparently connected with the period of the element by the very interesting relation of [[wikipedia:Geiger–Nuttall law|Geiger and Nuttall.[27] The intricate researches by which the complex series of transformations have been explained belong to the subject of Radioactivity and cannot be described here. From the point of view of isotopes it will be enough to consider the final results which are given in the two diagrams (Figs. 1 and 2).
In the first of these, which is due to Soddy,[28] the nuclear charge or Atomic number,[29] upon which all the chemical and spectroscopic properties of the elements depend, and which expresses its position in the periodic table, is indicated by a series of columns edged with thick fines sloping downwards to the right. The atomic weights are shown by fine lines sloping in the opposite direction. The fines corresponding to even atomic weights have been omitted to simplify the scheme. All elements lying in the same column will therefore be isotopes and all elements lying on the same fine sloping up to the right will be isobares. The α and β ray changes are shown by arrows and the period of decay of the elements indicated by times expressed in suitable units.
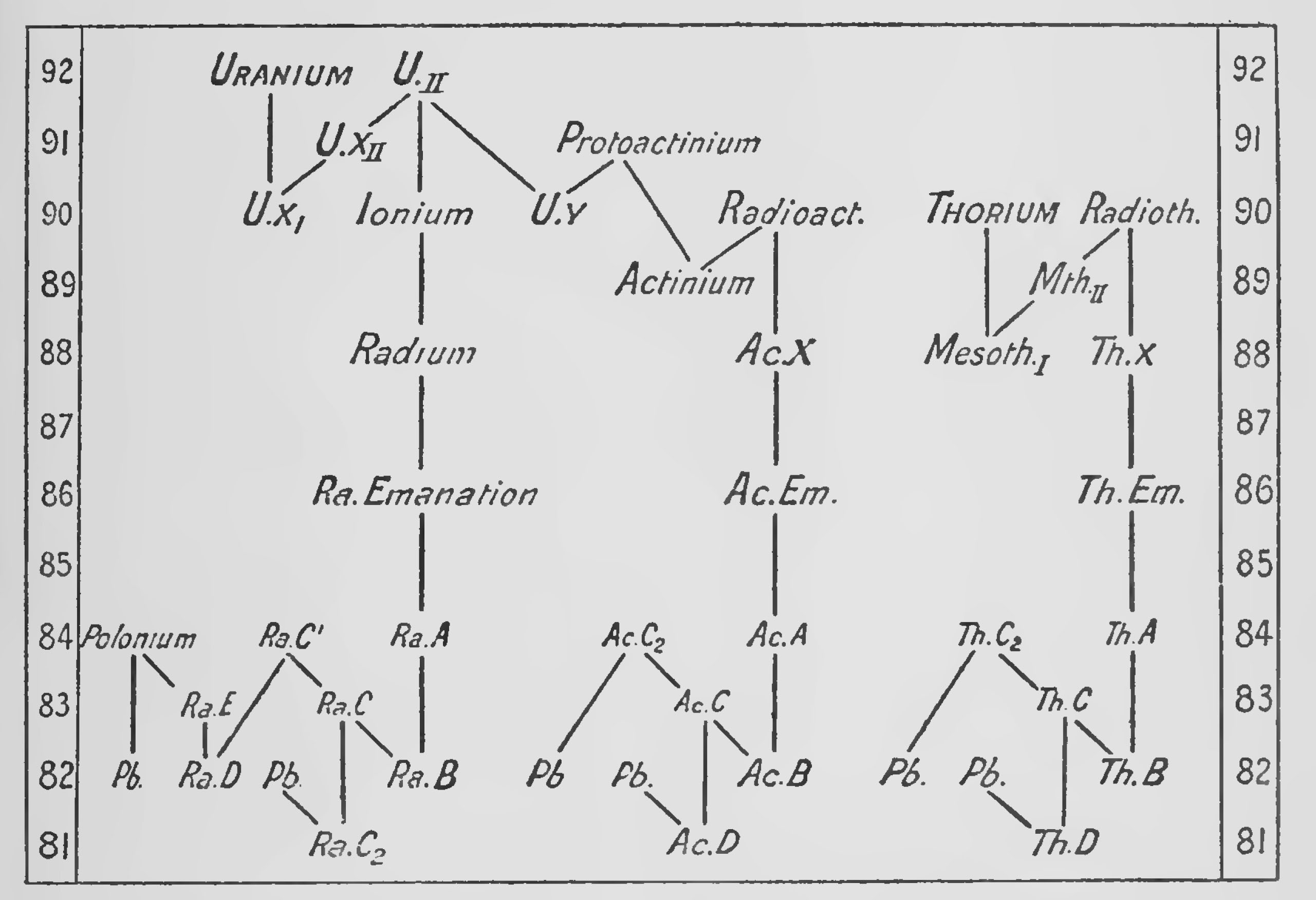
The second diagram[30] is arranged in a simple manner to show the general chains of transformation at a glance. In it the α and βray changes are plotted against atomic number and the other information omitted. On this diagram all elements lying on the same horizontal level will be isotopes. To take an example, uranium I which has an atomic number 92 and an atomic weight 238 loses one α particle and becomes uranium X, atomic number 90, atomic weight 234, This then gives off two β rays in succession, first becoming uranium X2 and then Uranium II. Uranium II has an atomic number 92 so that it is an isotope of uranium I. It has an atomic weight 234 so it is anisobare of uranium Xi and uranium X2. Uranium II can disintegrate by shooting off an a particle in two different ways ; about 8 per cent, of its atoms appear to form uranium Y, which is probably the parent substance of the actinium series. Disregarding this for the moment and following the main chain, 92 per cent, of the atoms of uranium II suffer an α ray change and are transformed into ionium, atomic weight 230, atomic number 90. Ionium loses an a particle and becomes radium, atomic weight 226. This by the same process changes to radium emanation, then to radium A, and then to radium B with atomic weight 214, We see that uranium II has lost 5 α particles in succession, thereby coming back 10 places, 92-82 in the periodic table, and its atomic weight has been reduced 20 units in the process. Radium B loses a β particle, becoming radium C which can disintegrate in two different ways. An extremely small proportion, 0.03 per cent., of its atoms undergo an α ray change to radium C2 which then loses a β particle and may become inactive lead of atomic weight 210. The vast majority of the atoms of radium C lose a β particle and form radium C. This next loses an a particle and becomes radium D, an active isotope of lead of atomic weight 210. Radium D now loses two β particles in succession, becoming radium E and then radium E, which is also called polonium. This finally undergoes its last a ray change and becomes inactive uranium lead of atomic weight 206.
The thorium and actinium chains can be followed on the diagrams in the same manner, but in the case of actinium the parent elements are not satisfactorily settled so that the atomic weights in this series are all doubtful.
10. The Atomic Weight of Lead
The theory of Isotopes of which Professor Soddy had proved himself so prominent an advocate and defender, received its most triumphant vindication, as far as it concerned the products of radioactivity, at the hands of the very chemists who had most reason to doubt its general apphcation, the specialists in the determination of atomic weights.
The charts of radioactive disintegration[31] show that the final product of every series is lead. If we take the main chain of the uranium-radium transformation this lead must have an atomic weight 206, for it has lost 5 alpha particles – each of weight 4 – since it was radium, and the atomic weight of radium is 226. On the other hand if we take the main thorium chain the lead end product must be 6 alpha particles lighter than thorium (232.15) and so should have an atomic weight about 208.
Now ordinary lead, from non-radioactive sources has an atomic weight 207.20, so Soddy[32] suggested in 1913 that the lead derived from minerals containing uranium but no thorium might have a smaller atomic weight than ordinary lead, and on the other hand the atomic weight of lead from minerals containing thorium but no uranium might be greater.
The first experiments were made by Soddy and Hyman[33] with a very small quantity of lead from Ceylon Thorite. This gave a perceptibly higher atomic weight than ordinary lead. Later a large quantity of the same mineral was available. The lead from this when carefully purified gave a density 0'26 per cent, higher than that of common lead. On the assumption that the atomic volumes of isotopes are equal this figure corresponds to an atomic weight of 207.74. A chemical atomic weight determination gave 207-694. A sample of the same lead was sent to Vienna where Professor Honigschmid, a well known expert in such matters, obtained from it a value 207.77 as a mean of eight determinations. These figures not only showed that thorium lead had a higher atomic weight than ordinary lead but also that their atomic volumes were identical, as expected from theory.[34]
At the same time as this work was in progress, the leading American authority on atomic weights, T. W. Richards of Harvard, started a series of investigations on lead derived from various radioactive minerals.[35] The samples of lead from uranium minerals all gave results lower than ordinary lead, as was expected, and one particularly pure specimen of uraniolead from Norwegian cleveite gave 206.08,[36] a very striking agreement with theory. The following table of properties is taken from his Presidential address to the American Association at Baltimore, December, 1918.
| Common Lead | Mixture Australian | Uranio-Lead | Percentage Difference | ||
|---|---|---|---|---|---|
| A | B | C | A--B | A--C | |
| Atomic weight | 207.19 | 206.34 | 206.08 | 0.42 | 0.54 |
| Density | 11.337 | 11.280 | 11.273 | 0.42 | 0.56 |
| Atomic volume | 18.277 | 18.278 | 18.281 | 0.01 | 0.02 |
| Melting point (absolute) | 600.53 | 600.59 | --- | 0.01 | --- |
| Solubility (of nitrate) | 37.281 | 37.130 | --- | 0.41 | --- |
| Refractive Index (nitrate) | 1.7815 | 1.7814 | --- | 0.01 | --- |
| Thermoelectric effect | --- | --- | --- | 0.00 | --- |
| Spectrum wave-length | --- | --- | --- | 0.00 | 0.00 |
In further confirmation Maurice Curie in Paris[37] reported 206.36 for a lead from carnotite, and a still lower figure, 206.046, was obtained by Honigschmid in Vienna for a lead from the very pure crystalised pitchblende from Morogoro. This is the lowest atomic weight found so far. The highest, 207.9, was also determined by Honigschmid for lead from Norwegian thorite.[38]
11. Atomic weights of Thorium and Ionium
Although the above results obtained with lead are far the most conclusive and important it is not the only element which affords direct experimental evidence of the different atomic weights of isotopes. The atomic weight of ionium, calculated by adding the weight of one alpha particle to the atomic weight of its product, radium, is 230, whereas that of thorium, its isotope, is slightly above 232. Joachimsthal pitchblende contains hardly any thorium so that an ionium-thorium preparation separated by Auer von Welsbach from 30 tons of this mineral might be regarded as containing a maximum concentration of ionium. On the other hand the period of thorium is about 105 times longer than that of ionium so that it was doubtful if even in this preparation there would be enough ionium to show a difference in atomic weight. Honigschmid and Mlle. Horovitz have made a special examination of this point, first redetermining as accurately as possible the atomic weight of thorium and then that of the thorium-ionium preparation from pitchblende. They found 232.12 for the atomic weight of thorium, and by the same careful method 231.51 for that of the thorium-ionium.
12. Use of radioactive isotopes as indicators
Consider an inactive element A which has a radioactive isotope B. If these are mixed together in any proportions no chemical or physical process known is capable of altering the ratio of the proportions of this mixture to any measurable extent. Now the radioactive methods of detecting and measuring B are many millions of millions of times more delicate than the chemical methods of detecting and measuring A, so that by mixing with A a small quantity of B we can trace its presence far beyond the limits of chemical analysis. We have, as it were, marked the atoms of A with an indelible label so that the minutest trace of the element can be measured with ease and certainty.
By this powerful and novel device, which has been developed by G. Hevesy[39] 10-9 gr. of lead can be determined quantitatively and solution concentrations can be dealt with down to 10-14 of normal. By adding radium D to the lead salt and estimating it electroscopically the solubility of lead sulphide and chromate, and the amount of lead chloride carried down in a silver chloride precipitate, may readily be determined.
Recently, by the same principle, it has been shown that a free exchange of the metallic atom among the competing acid radicles occurs for ionised, but not for non-ionised, compounds. The general method was to mix solutions of two different compounds of lead in equimolecular proportions, the one compound only being "activated" by presence of thorium-B (which is isotopic with lead), and to determine the activity of the lead in the less soluble compound crystallising out. When active lead nitrate and inactive lead chloride are dissolved in molecular proportion in boiling pyridine, the lead in the lead chloride crystalHsing out is half as active as the lead in the original lead nitrate, but when such an active lead salt is so mixed with an organic compound of lead, such as lead tetraphenyl or diphenyl nitrate, in suitable solvents, no interchange of lead occurs, and the active lead salt retains its original activity. This constitutes something like a direct proof of the ionic dissociation theory and of the current views as to the difference between the nature of chemical union in electrolytes and non-electrolytes. When the acetates of quadrivalent activated lead and of bivalent inactive lead are mixed in glacial acetic acid, the activity of the first compound, after crystallising out from the mixture, is reduced to one half. This indicates, since the two lead ions differ only by two electrons, a free interchange of electrons between them and a dynamic equilibrium between ions and electrons and between free electrons and the electrodes in electrolysis.[40]
Isotopes can also be used to determine the velocity of diffusion of molecules among themselves.[41] The rate of diffusion is dependent on the molecular diameter, and not on the mass, so that a radioactive element diffusing among the inactive molecules of its isotope affords a means of investigating this otherwise insoluble problem. The experiment has been tried with molten lead. At the bottom of a narrow vertical tube was placed a layer of lead rendered active by the presence of thorium-B, and above it a layer three times the height of common lead. The whole was kept at 340° for several days. After cooling, the cylinder was cut into four equal lengths, each melted and hammered into foil, and the concentration of thorium-B in each determined by alpha ray measurements. Values for the diffusion coefficient between 1.77 and 2.54 per sq. cm. per day, with a mean of 222 in seventeen experiments, were obtained. On certain theories of physical chemistry this corresponds with a diameter of the lead molecule between 0.78 and I1.16 x 10-8 cm., according to the formulae used to connect the two quantities. The value found by similar theories when reduced to a temperature of 18° and for a fluid of the viscosity of water, becomes 2.13. Since the value for lead ions diffusing in aqueous solutions is 0.68, this indicates that the molecular diameter in the case of metallic lead is only a third of that in the case of the ion, and shows that the latter is probably hydrated.[42]
13. Classification of the radioactive isotopes
It is clear that the relations between isotopes formed by radioactive disintegrations need not necessarily have the same simple form as those subsisting between isotopes of the inactive elements. Neuberger,[43] using the nucleus model of the radioelements proposed by Lise Meitner,[44] suggests that the radioactive isotopes may be divided into three or even four classes.
- (1) Isotopes of the first class are those which possess only the same nuclear charge and the same arrangement of outer electrons such as radium and mesothorium I.
- (2) Isotopes of the second class have, in addition, the same nuclear mass, that is to say the same atomic weight, and the same total number of nuclear "building stones." Examples of this class are ionium and uranium Y.
- (3) Isotopes of the third class still possess the same number of each nuclear building stone, but they have a different arrangement of these in the atomic nucleus, and thus possess different chances of disintegrating, such as Radium D and Actinium B.
- (4) Isotopes of the fourth class would be those possessing the same arrangement of nuclear building stones in the atomic nucleus, and thus the same probability of disintegrating. Such isotopes actually exist, but we have no available means of distinguishing between them. Hence we cannot at present designate them definitely as isotopes. Examples of these are radium C2 and actinium D.
References
- ↑ Boltwood, Amer. J. Sci, 22, 537, 1906;[1] 24, 370, 1907.[2]
- ↑ Keetman, Jahr. Radioactivitat, 6, 269, 1909.
- ↑ A. von Welsbach, Wien. Ber. iia, 119, 1011, 1910.
- ↑ Marckwald, Ber. d. Chem. Oes., 40, 3420, 1910.
- ↑ Soddy, Trans. Chem. Soc, 99, 72, 1911.
- ↑ Hahn and Meitner, Physikal. Zeitsch., 11, 493, 1910.
- ↑ Soddy, Chem. Soc. Ann. Rep., 285, 1910.
- ↑ Russell and Rossi, Proc. Roy. Soc, 77A, 478, 1912.
- ↑ Soddy, Chem. News, Feb. 28, 1913.
- ↑ V. p. 92.
- ↑ G. Hevesy, Phil. Mag., 23, 628, 1912 ; Physikal. Zeitsch. 15, 672, 715, 1912.
- ↑ V. p. 19.
- ↑ Paneth and Hevesy, Sitzungber. K. Akad. Wiss. Wien, iiA, 123, 1037, 1913.
- ↑ Rutherford and Andrade, Phil. Mag. 27, 854, 1914.
- ↑ V. p. 93.
- ↑ V. Stewart, Recent Advances in Physical and Inorganic Chemistry, Longmans, 1919.
- ↑ Soddy, The Chemistry of the Radio Elements, 29, First Edition, Longmans, 1911.
- ↑ Russell, Chem. News, Jan, 31, 1913,
- ↑ Fajans, Physikal. Zeitsch. Feb. 15, 1913.
- ↑ Hevesy, Physikal. Zeitsch. Jan. 15, 1913.
- ↑ Fleck, Trans. Chem. Soc. 103, 381, 1052, 1913.
- ↑ V. p. 93.
- ↑ Stewart, Phil. Mag. 36, 326, 1918.[3]
- ↑ V. p. 91.
- ↑ V. p. 77.
- ↑ V. Table p. 106.
- ↑ Rutherford, Radioactive Substances and their Radiations, p. 607, Cambridge, 1913.
- ↑ Soddy, Trans. Chem. Soc, U5, 16, 1919.
- ↑ V. p. 93.
- ↑ Darwin, Nature, 106, 82, 1920.[4]
- ↑ P. 14.
- ↑ 1 Soddy, Ann. Rep. Chem. Soc, 269, 1913.
- ↑ 2 Soddy and Hyman, Trans. Chem. Soc, 105, 1402, 1914.
- ↑ Soddy, Roy. Ins., May 18, 1917.
- ↑ Richards and Lembert, J. Amer. Chem. Soc, 36, 1329, 1914. [5]
- ↑ 5 Richards and Wadsworth, J. Amer. Chem. Soc, 38, 2613, 1916.
- ↑ M. Curie, Compt. Bend., 158, 1676, 1914.
- ↑ Honigschmid, Zeit. Elektrochem., 24, 163, 1918 ; 25, 91, 1919.
- ↑ Hevesy, Brit. Assoc, 1913 ; Chem. News, Oct. 13, 166, 1913.
- ↑ G. Hevesy and L. Zechmeister, Ber., 53B, 410, 1920 ; Zeitsch. Elektrochem. 26, 151, 1920.
- ↑ J. Groh and Hevesy, Ann. Physik., iv., 63, 85, 1920.
- ↑ Soddy, Ann. Rep. Chem. Soc, 227, 1920.
- ↑ Neuburger, Nature, 108, 180, 1921.
- ↑ Meitner, Die Naturwissenschaften, 9, 423, 1921.
Francis William Aston (1922), Isotopes, ISBN 978-1016732383, Internet Archive.
