McLafferty rearrangement: Difference between revisions
No edit summary |
|||
| (27 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{ | {{Final | ||
|acronym= | |||
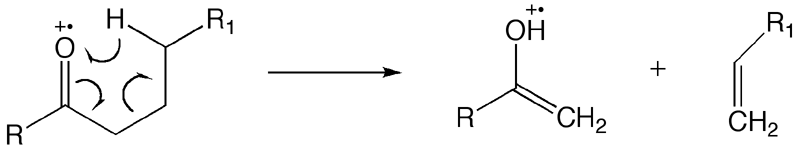
|def=Rearrangement reaction involving transfer of a hydrogen atom via a six-member transition state to the formal radical/charge site from a carbon atom four atoms distant from the charge/radical site (the γ-carbon); subsequent rearrangement of electron density can lead to expulsion of an [[wikipedia:olefin|olefin]] molecule. | |||
:Note: Originally applied to [[wikipedia:ketone|ketone]] [[molecular ion]]s where the charge/radical site is the carbonyl oxygen, but is now more widely applied. | |||
|rel= | |||
|ref={{obgb}} | |||
}} | }} | ||
{{wplink}} | |||
---- | |||
<!-- Orange Book --> | |||
{{orange| | |||
This is an example of a rearrangement reaction and is defined as β - cleavage with concomitant specific transfer of a γ - hydrogen atom in a six-membered transition state in mono-unsaturated systems, irrespective of whether the rearrangement is formulated by a radical or by an ionic mechanism and irrespective of with which fragment the charge stays. | This is an example of a rearrangement reaction and is defined as β - cleavage with concomitant specific transfer of a γ - hydrogen atom in a six-membered transition state in mono-unsaturated systems, irrespective of whether the rearrangement is formulated by a radical or by an ionic mechanism and irrespective of with which fragment the charge stays. | ||
}} | |||
<!-- Gold Book --> | |||
{{gold| | |||
http://goldbook.iupac.org/M03772.html | |||
β-Cleavage with concomitant specific transfer of a γ-hydrogen atom in a six-membered transition state in mono-unsaturated systems, irrespective of whether the rearrangement is formulated by a radical or an ionic mechanism, and irrespective of the position of the charge. | β-Cleavage with concomitant specific transfer of a γ-hydrogen atom in a six-membered transition state in mono-unsaturated systems, irrespective of whether the rearrangement is formulated by a radical or an ionic mechanism, and irrespective of the position of the charge. | ||
'''Source''': | |||
[[Orange Book]] p. 207 | |||
}} | |||
: | ==Gallery== | ||
[[File:McLafferty rearrangement.gif|thumb|600 px|center|An example of the McLafferty Rarrangement]] | |||
[[File:McLafferty rearrangement.png|thumb|600 px|center| McLafferty rearrangement | |||
from {{doi}}10.1016/j.jasms.2004.05.009 p. 19]] | |||
[[Category:Reactions]] | [[Category:Reactions]] | ||
Latest revision as of 20:40, 3 July 2025
| IUPAC RECOMMENDATIONS 2013 |
| McLafferty rearrangement |
|---|
Rearrangement reaction involving transfer of a hydrogen atom via a six-member transition state to the formal radical/charge site from a carbon atom four atoms distant from the charge/radical site (the γ-carbon); subsequent rearrangement of electron density can lead to expulsion of an olefin molecule.
|
| Related Term(s): |
| Reference(s):
IUPAC. Analytical Division. Compendium of Analytical Nomenclature (the Orange Book). Definitive Rules, 1979. Compiled by J. Inczdy, T. Lengyel, A. M. Ure. Blackwell Scientific Publications, Oxford (1997). On-line corrected version: http://www.iupac.org /publications/analytical compendium (2000). IUPAC. Compendium of Chemical Terminology, 2nd ed. (the Gold Book). Compiled by A. D. McNaught and A.Wilkinson. Blackwell Scientific Publications, Oxford (1997). XML on-line corrected version: http://goldbook.iupac.org (2006-) created by M. Nic, J. Jirat, B. Kosata; updates compiled by A. Jenkins. |
| From Definitions of Terms Relating to Mass Spectrometry (IUPAC Recommendations 2013); DOI: 10.1351/PAC-REC-06-04-06 © IUPAC 2013. |
|
This term has a corresponding Wikipedia article: Wikidata page for McLafferty rearrangement |
Orange Book
| ORANGE BOOK DEFINITION
IUPAC. Analytical Division. Compendium of Analytical Nomenclature (the Orange Book). Definitive Rules, 1979 (see also Orange Book 2023) |
| McLafferty rearrangement |
|---|
|
This is an example of a rearrangement reaction and is defined as β - cleavage with concomitant specific transfer of a γ - hydrogen atom in a six-membered transition state in mono-unsaturated systems, irrespective of whether the rearrangement is formulated by a radical or by an ionic mechanism and irrespective of with which fragment the charge stays. |
| IUPAC 1997 Orange Book Chapter 12 |
| Index of Orange Book Terms |
Gold Book
| GOLD BOOK DEFINITION
IUPAC. Compendium of Chemical Terminology, 2nd ed. (the Gold Book). Compiled by A. D. McNaught and A.Wilkinson. Blackwell Scientific Publications, Oxford (1997). |
| McLafferty rearrangement |
|---|
|
http://goldbook.iupac.org/M03772.html β-Cleavage with concomitant specific transfer of a γ-hydrogen atom in a six-membered transition state in mono-unsaturated systems, irrespective of whether the rearrangement is formulated by a radical or an ionic mechanism, and irrespective of the position of the charge. Source: Orange Book p. 207 |
| IUPAC Gold Book |
| Index of Gold Book Terms |
Gallery